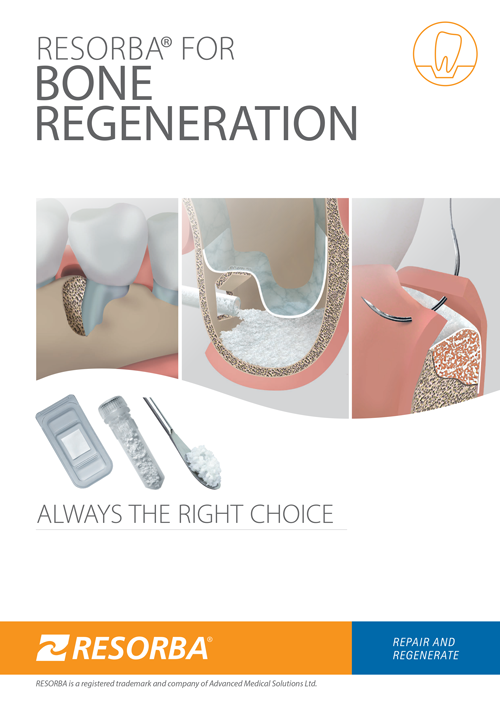
ORAL SURGERY
BONE REGENERATION
Bone and soft tissue regeneration is routine and a common method of rebuilding the jawbone. Since decades it has been proven predictable outcomes and success. Trained oral surgeons, implantologists and periodontists are thus able to regenerate
defects in most cases and replace lost teeth. However, certain cases and the increasing aesthetic demands of patients often challenge know-how and skills of the dentist.
A precise anamnesis and diagnosis of the patients, as well as consideration of their
general state of health, age and gender, are essential so that treatment techniques can
be adapted accordingly in order to promote the regeneration process.
The blood supply in the surgical area is of prime importance – without rapid wound
healing and recovery of blood circulation, an uneventful and complication-free healing
process is not possible. Our product range offers dentists a comprehensive selection of cutting-edge products to meet the requirements of modern oral surgery.

DENTAL COLLAGEN MEMBRANES
DENTAL BONE SUBSTITUTES
Equine collagen, native crosslinked
- PARASORB RESODONT®
- PARASORB RESODONT® Forte
Porcine Tissue, chemically crosslinked
- RESORBA® EasyFlex
- PARASORB RESODONT® Flex
- RESORBA® Xenogenic Bone Graft
Biphasic Calcium Phosphate (MBCP® Technology),
60% HA / 40% ß-TCP & 20% HA / 80% β-TCP
- RESORBA® Synthetic Bone
DENTAL COLLAGEN MEMBRANES
Equine collagen, native crosslinked
- PARASORB RESODONT®
- PARASORB RESODONT® Forte
Porcine Tissue, chemically crosslinked
- RESORBA® EasyFlex
- PARASORB RESODONT® Flex
DENTAL BONE SUBSTITUTES
- RESORBA® Xenogenic Bone Graft
Biphasic Calcium Phosphate (MBCP® Technology), 60% HA / 40% ß-TCP & 20% HA / 80% β-TCP
- RESORBA® Synthetic Bone
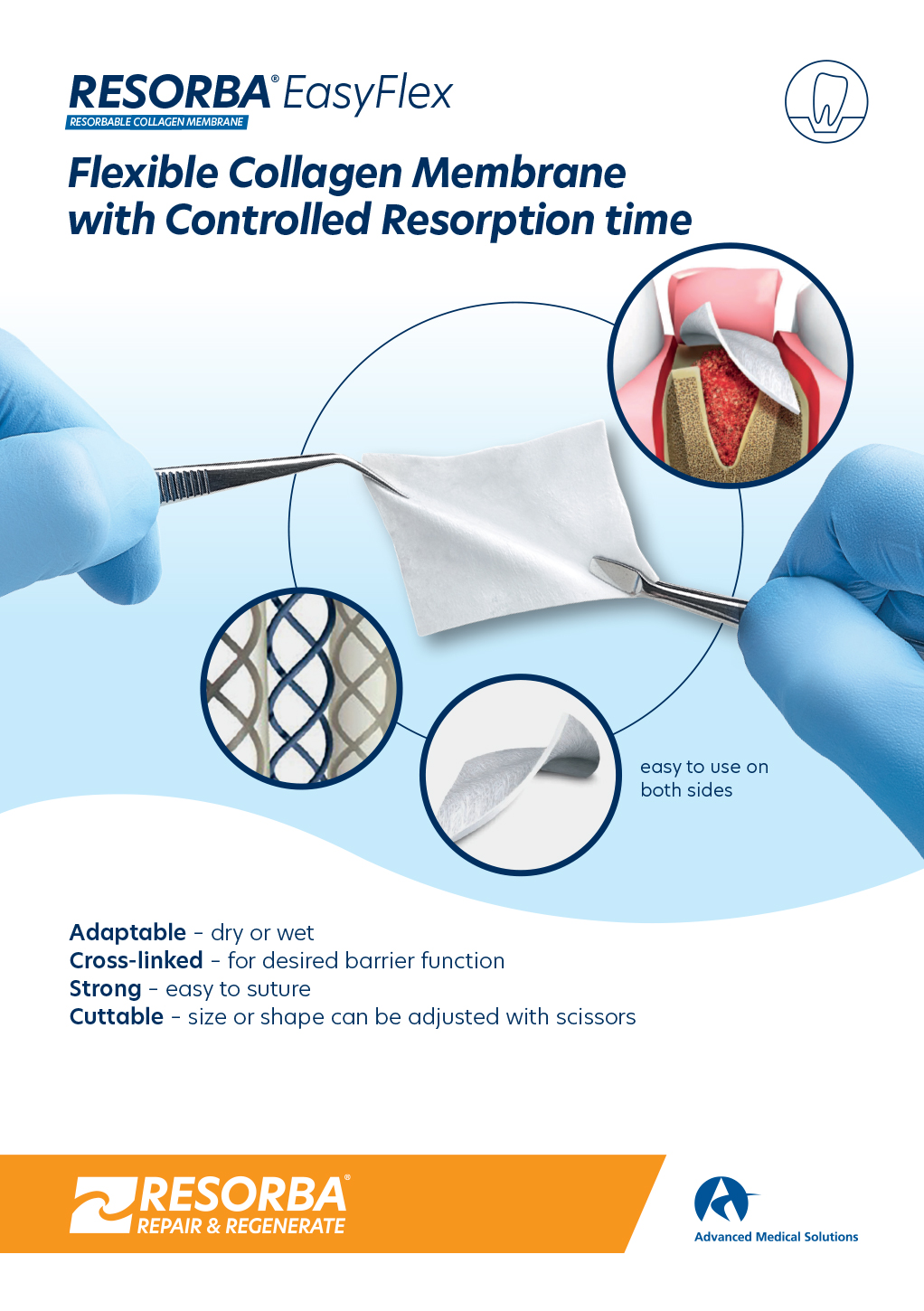
WHY COLLAGEN?
Completely absorbable
This means that there is no need for a second operation such as is required with non-resorbable materials. The naturally-structured collagen fibrils activate clotting like endogenous collagen.
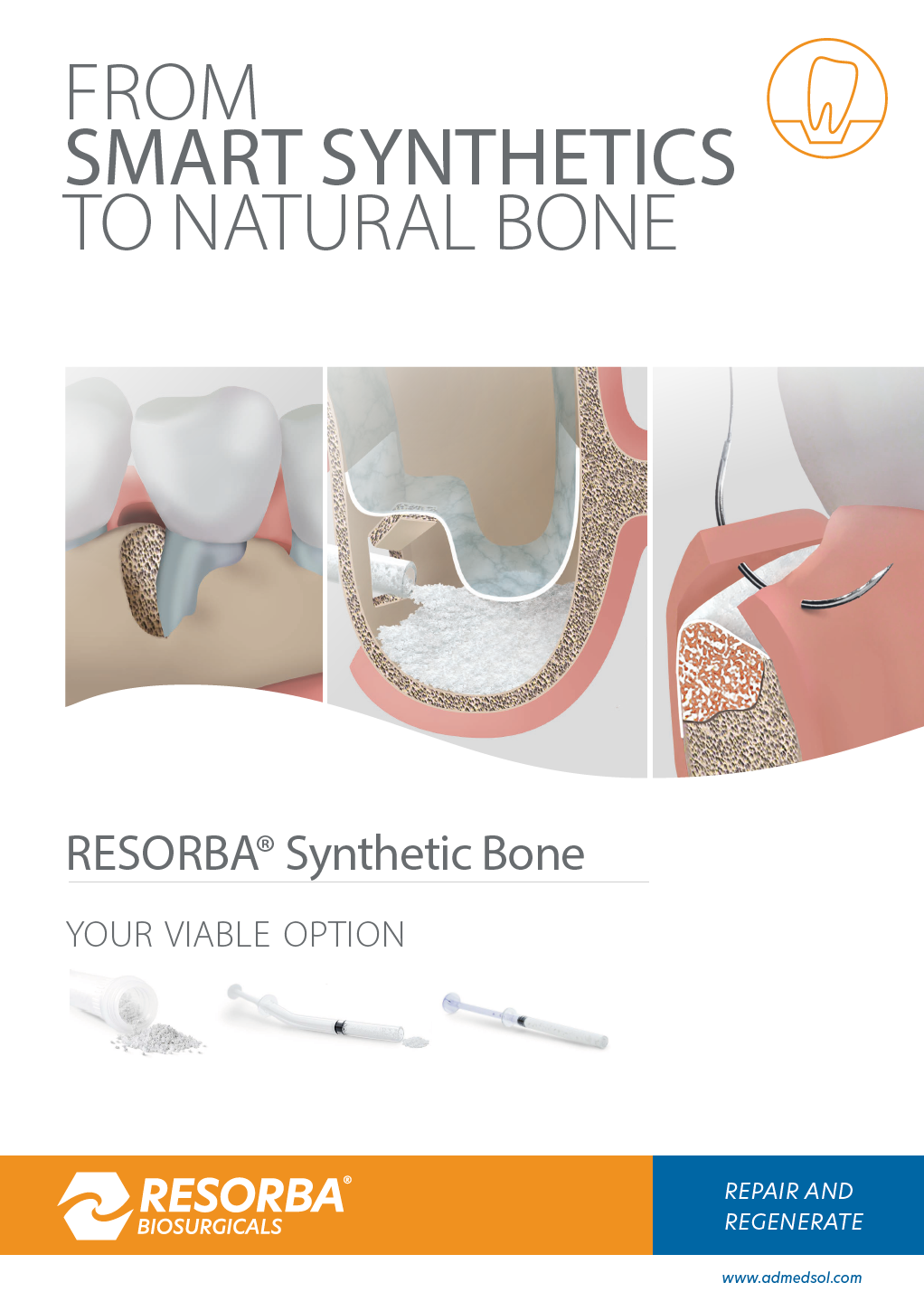
WHY SYNTHETIC BONE?
Synthetic bone is an unlimited source and a safe and proven scaffold for bone augmentation. The mixture of Hydroxyapatite (HA) and Beta-Tricalcium Phosphate (ß-TCP) is best investigated.
WHY XENOGENIC BONE?
Ideal scaffold
Xenogenic bone grafts are widely used since decades. The osteoconductive character grants for a predictable bone regeneration and long lasting volume stability. It is used in any oral bone regeneration procedure.
MEMBRANES
ABSORBABLE COLLAGEN BARRIER MEMBRANES
3 Different Types
PARASORB RESODONT®
- 1 cm2 contains 2.38 – 3.22 mg native equine collagen fibrils
- Will be absorbed within 6 – 8 weeks
- Shelf life: 5 years
PARASORB RESODONT® Forte
- Highly concentrated collagen to extend barrier function
- 1 cm2 contains 6.0 – 8.0 mg native equine collagen fibrils
- Will be absorbed within 12 – 16 weeks
- Shelf life: 5 years
PARASORB RESODONT® Flex
- Porcine Peritoneum Tissue
- Will be absorbed within 12 – 16 weeks
- Shelf life: 3 years
RESORBA® EasyFlex
- Porcine dermis origin
- 12 weeks safe barrier
- Shelf life: 3 years
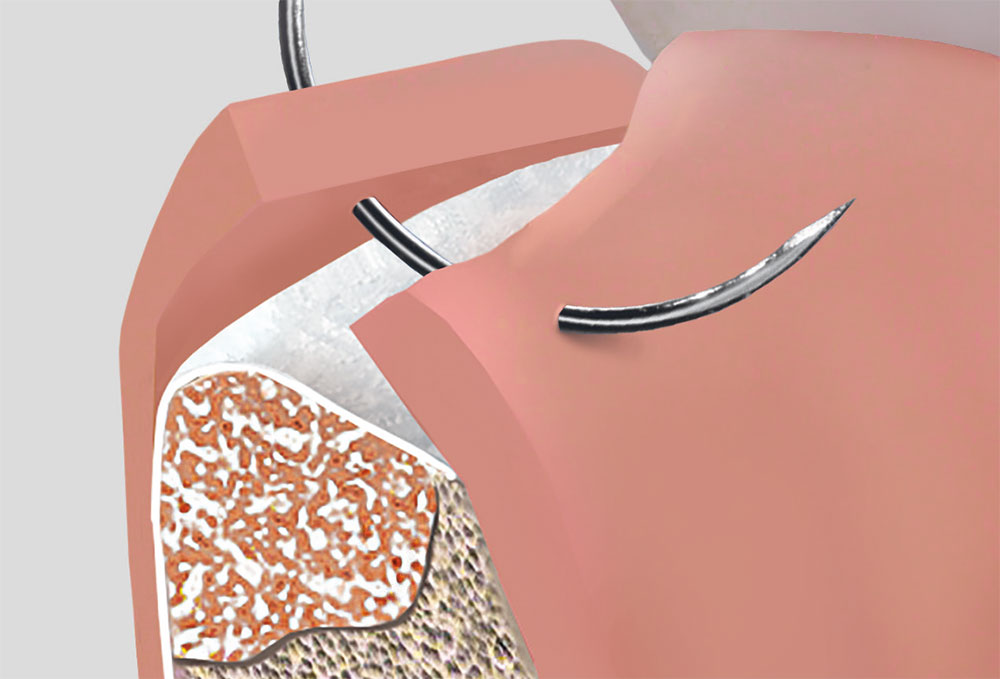


BONE SUBSTITUTES
ABSORBABLE BONE SUBSTITUTES FOR BONE AUGMENTATION PROCEDURES
2 Different Types
RESORBA® Xenogenic Bone Graft
- Porcine Xenograft Particulate
- Processed using optimal heat to maintain interconnecting macro and micropores (up to 95% void space)
- Shelf life: 3 years
RESORBA® Synthetic Bone
- Biphasic Calcium Phosphate (MBCP® Technology), 20% HA / 80% ß-TCP
- 70% porosity thanks to interconnected macro and micropores
- Shelf life: 5 years
PRODUCT CHARACTERISTICS
MEMBRANES
No fixation required
Easy to adapt
Easy to trim
Complete integration into the surrounding tissue
Fully absorbable
Reliable barrier function
BONE SUBSTITUTES
Osteoconductive
Interconnecting pores supporting the formation of new bone
Easy to handle
Fully absorbable
INDICATIONS AND PRODUCT RECOMMENDATIONS
MEMBRANES
BONE SUBSTITUTES
PARASORB RESODONT®
- Schneiderian membrane coverage in sinus lift procedures
- Alveolar Ridge Preservation (ARP) with closed healing
PARASORB RESODONT® Forte
- Guided Bone Regeneration procedures (GBR)
- Guided Tissue Regeneration procedures (GTR)
- Block-graft procedures
- Ridge-split procedures
- Sinus lift lateral window coverage
- Alveolar Ridge Preservation (ARP) with closed and open healing
- Management of antro-oral communication
- Oral and maxillofacial trauma
PARASORB RESODONT® Flex
- Guided Bone Regeneration procedures(GBR)
- Guided Tissue Regeneration procedures (GTR)
- Sinus lift lateral window coverage
- Alveolar Ridge Preservation (ARP)
- Augmentation around dental implants
- Periodontal Regeneration procedures
RESORBA® Xenogenic Bone Graft
- Alveolar Ridge Preservation procedures (ARP)
- Bone defect stabilization
- Guided Bone Regeneration procedures (GBR)
- Sinus-lift procedures
- Ridge-split procedures
- Periodontal Regeneration procedures
RESORBA® Synthetic Bone
- Alveolar Ridge Preservation procedures (ARP)
- Bone defect stabilization
- Guided Bone Regeneration procedures (GBR)
- Sinus-lift procedures
- Ridge-split procedures
- Periodontal Regeneration procedures
MEMBRANES
PARASORB RESODONT®
- Schneiderian membrane coverage in sinus lift procedures
- Alveolar Ridge Preservation (ARP) with closed healing
PARASORB RESODONT® Forte
- Guided Bone Regeneration procedures (GBR)
- Guided Tissue Regeneration procedures (GTR)
- Block-graft procedures
- Ridge-split procedures
- Sinus lift lateral window coverage
- Alveolar Ridge Preservation (ARP) with closed and open healing
- Management of antro-oral communication
- Oral and maxillofacial trauma
PARASORB RESODONT® Flex
- Guided Bone Regeneration procedures (GBR)
- Guided Tissue Regeneration procedures (GTR)
- Sinus lift lateral window coverage
- Alveolar Ridge Preservation (ARP)
- Augmentation around dental implants
- Periodontal Regeneration procedures
BONE SUBSTITUTES
RESORBA® Xenogenic Bone Graft
- Alveolar Ridge Preservation procedures (ARP)
- Bone defect stabilization
- Guided Bone Regeneration procedures (GBR)
- Sinus-lift procedures
- Ridge-split procedures
- Periodontal Regeneration procedures
RESORBA® Synthetic Bone
- Alveolar Ridge Preservation procedures (ARP)
- Bone defect stabilization
- Guided Bone Regeneration procedures (GBR)
- Sinus-lift procedures
- Ridge-split procedures
- Periodontal Regeneration procedures
RANGE
ABSORBABLE COLLAGEN MEMBRANES
| PRODUCT | REF | Size | Content Box |
|---|---|---|---|
| PARASORB RESODONT® | RD2502 | 22 x 25 mm | 1 membrane |
| RD3503 | 32 x 25 mm | 1 membrane | |
| RD0703 | 64 x 25 mm | 1 membrane | |
| PARASORB RESODONT® Forte | RDF1502 | 16 x 25 mm | 1 membrane |
| RDF2502 | 22 x 25 mm | 1 membrane | |
| RDF3503 | 32 x 25 mm | 1 membrane | |
| RDF0703 | 64 x 25 mm | 1 membrane | |
| PARASORB RESODONT® Flex 1 | RDC1520 | 15 x 20 mm | 1 membrane |
| RDC2030 | 20 x 30 mm | 1 membrane | |
| RDC3040 | 30 x 40 mm | 1 membrane | |
| RESORBA® EasyFlex 2 | SB0701EZC1525 | 15 x 25 mm | 1 membrane |
| SB0901EZC1525 | 15 x 25 mm | 2 membranes | |
| SB0702EZC2030 | 20 x 30 mm | 1 membrane | |
| SB0703EZC3040 | 30 x 40 mm | 1 membrane |
2 Manufacturer: Biomatlante SA, ZA Les Quatre Nations, 5 rue Edouard Belin, 44360 Vigneux de Bretagne, France
PORCINE XENOGRAFT PARTICULATE
| PRODUCT | REF | Particle Size | Content Box |
|---|---|---|---|
| RESORBA® Xenogenic Bone Graft2 | XBS050 | 0.25 mm – 1.0 mm | 1 jar, 0.5 cc |
| XBS100 | 0.25 mm – 1.0 mm | 1 jar, 1.0 cc | |
| XBS200 | 0.25 mm – 1.0 mm | 1 jar, 2.0 cc | |
| XBS400 | 0.25 mm – 1.0 mm | 1 jar, 4.0 cc | |
| XBL100 | 1.0 mm – 2.0 mm | 1 jar, 1.0 cc | |
| XBL200 | 1.0 mm – 2.0 mm | 1 jar, 2.0 cc |
2 Manufacturer: Collagen Matrix, Inc. · 15 Thornton Road · Oakland, NJ 07436, USA
BIPHASIC CALCIUM PHOSPHATE (MBCP® TECHNOLOGY) 60% HA / 40% ß-TCP
| PRODUCT | REF | Particle Size | Content Box |
|---|---|---|---|
| RESORBA® Synthetic Bone3 | SB0401G50 | 0.5 mm – 1.0 mm | 1 vial, 0.5 cc |
| SB9901G01 | 0.5 mm – 1.0 mm | 1 vial, 1.0 cc | |
| SB9902G02 | 1.0 mm – 2.0 mm | 1 vial, 2.0 cc | |
| SB0401GS50 | 0.5 mm – 1.0 mm | 1 syringe, 0.5 cc |
3 Manufacturer: Biomatlante SA, ZA Les Quatre Nations · 5, Rue Edouard Belin · 44360 Vigneux de Bretagne, France
Biomatlante SA is part of Advanced Medical Solutions Group plc.
DENTAL
DOWNLOADS
View all Resorba® videos, factsheets and resources:
Discover Resorba®
Our Product Range
AMS Group
Tools & Social
Discover Resorba®
Our Product Range
AMS Group
Tools & Social
Resorba is a registered trademark of RESORBA Medical GmbH. All rights reserved
Disclaimer | Accessibility | Cookies | Privacy Policy | Sitemap
Copyright © RESORBA Medical GmbH | Design by Lumisi